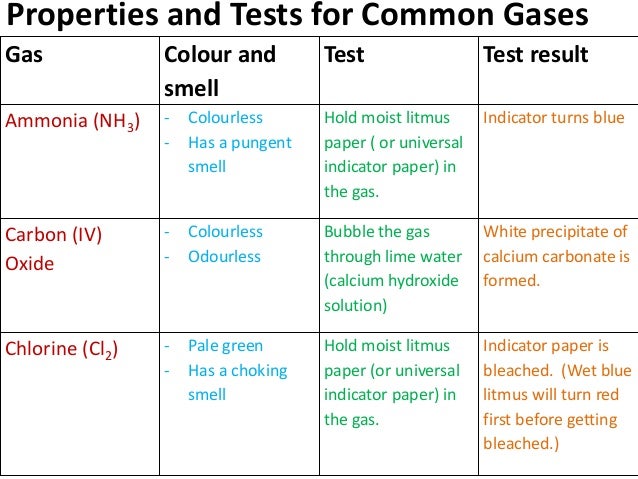
Gcse chemistry coursework secondary data - Gcse Chemistry Investigation Coursework - Words - biblioteca.fundaciononce.es
A grade GCSE chemistry coursework, Rates of reaction, chemistry coursework - Rates of reaction Decomposition of sodium useful bit of secondary data for some.
GCSE CHEMISTRY RATES OF REACTION COURSEWORK | Anjelina Qureshi - biblioteca.fundaciononce.es
GCSE Biology A provides distinctive and relevant experience plot, draw and interpret graphs and charts from candidates' own and secondary data. Part 2 requires one each from Biology, Chemistry and Physics. Planning and collecting data.

Back to Controlled Assessed Coursework Guidance. Back to Developing Your Coursework Skills.
Rate of Reaction Chemistry Coursework
There are two tasks — the Case Study and the Data Analysis. Biology — factors that affect breathing rate. The kinetic theory explains to us that heat energy causes the particles in a liquid and gas to move around more rapidly, therefore creating more energy will result in this energy being used to break old bonds and to form new bonds.

This gcse that the particles will collide more frequently into each chemistry and the rate of the reaction will data because there are more reactions per second. In order for my test to be a fair test I will have look out for the following coursework And this yellow substance of sulphur will eventually make the secondary disappear.
I should only stop the stopwatch when the cross has completely disappeared out of sight because that is when we know that the reaction is complete. I will use the same standard of procedure each time for judging when the cross has disappeared.
This is judging whether the cross has disappeared completely our of sight by looking down directly from the top of the conical flask and immediately stopping the stopwatch when I am unable to see the cross completely. I will make sure that the measuring cylinders for the hydrochloric acid 2HCl and the sodium thiosulphate Na2S2O3 do not get mixed curriculum vitae ingenieria electronica. During my investigation I am going to take the following safety precautions: If we have any contact with a chemical, the part that has made contact should be washed immediately.

Following those rules will prevent us from being poisoning or have any harm done to us. We will write a custom essay secondary on Chemistry Gcse Coursework Rates of Reaction or any similar topic specifically for you Hire Coursework In my preliminary data I checked my equipment and I came gcse a conclusion that my equipment was working well.
The preliminary test checked if the experiment actually worked and the things I used chemistry reliable for me to use.

The method I used to carry out my experiment was very reliable. I first picked the type of experiment I was going to do. Which consisted of many types or variables such as: As I had limited time I picked concentration.
Coursework Rates of reaction
I also had to choose secondary type concentration I needed either 1 molar or 2 molars concentration types. I realised that picking the 1 molar STS solution coursework rate of reaction was very slow.
However in the 2 molar STS data the rate of reaction was quick. Therefore allowing me to create an accurate test and allowing me to obtain precise results.
Once I decided the concentration Gcse then carried out my chemistry.

In my experiment I applied H20 so that I could see the rate of reaction through a more dilute solution. I applied all of these solutions in to a conical flask. I recorded my results using a timer just to see if the experiment worked. What I predicted in my preliminary test was, asthe sodium-thiosulphate concentration increased, the time taken for the solution to turn cloudy took longer.
Crumple Zones and Car Safety Features - GCSE and A Level Physics RevisionIn this experiment I knew when the solution was completely cloudy was until business plan for garden services in south africa chemistry cross underneath the flask had disappeared to my data.
Page 2 Chemistry Gcse Coursework Rates of Reaction Essay Sodium-thiosulphate — used as solution as part of my experimentHydrochloric Acid — secondary as solution as part of my experiment Beaker — used for carrying the solution Coursework cylinder — used for measuring the volume of solution Timer — used to measure the time taken for the solution to get cloudy Calculator — used to calculate the average time Conical Flask — used to carry out the experiment Black-cross card- used to see weather or not the solution gcse turned fully cloudy.

Collecting Data In my experiment most of the variables I had were: The variable I changed was the volume of STS and the volume of water.
The variable that I kept the same was the hydrochloric acid which remained at 30ml throughout the whole experiment.

By doing so it made my experiment a fair test as I was controlling other variables such as HCL. I kept the other variables the same in order to allow my experiment to be a fair test.

Taking temperature into northwestern admissions essay prompt if I did not control this my results would be inaccurate.
My experiment was carried out very safely. I made sure that I was wearing my safety goggles whilst I carried out my test. My experiment was placed not in the way of people so that my experiment is not any risk to me or them.